ABOUT THE OFFICE
Technology transfer is the process by which USU’s knowledge, facilities, and capabilities are translated to benefit the Warfighter and to fulfill public and private needs.
The Office of Research and Technology Applications (ORTA) serves USU researchers as an agreements, licensing, patenting, and technology commercialization office for research and innovations at USU.
We promote and support the transfer of USU’s research outcomes for the benefit of USU, the Warfighter, and the nation. Through the ORTA, USU faculty, researchers, and students have their discoveries further developed and translated for the public good.
We coordinate Cooperative Research and Development Agreements (CRADAs), Material Transfer Agreements (MTAs), Nondisclosure Agreements (NDAs), and other technology transfer transactions on behalf of USU researchers. USU researchers are supported in intellectual property protection, marketing, and technology licensing, serving to promote the development and commercialization of USU’s innovative medical technologies and discoveries.
The ORTA maintains a presence in the university laboratories to ensure ongoing education in technology transfer for researchers and the prompt identification of intellectual property. Investigators who are familiar with the process are better prepared to understand the potential novelty of their research and the pipeline to commercialization.
We forge close ties between USU and industry by providing opportunities for exchange of information and materials and facilitating collaborations among researchers at USU, HJF, other universities and foundations, government entities, and private industry. Licensing revenue from successful technology transfer is shared among the inventors and USU to help support technology transfer services and to provide additional research funding for USU.
“Advancing technologies from bench to bedside to support military medicine and improve health worldwide.”
Tech Transfer Successes
We have helped USU researchers protect their inventions and discoveries – USU had 685 active patents in 2018. These include intellectual property coverage ranging from biomarkers to detect traumatic brain injury and post-traumatic stress disorder; radiation detection and countermeasures; use of antibodies to detect prostate cancer. Notable success stories of the tech transfer of USU intellectual property include the RSV vaccine, Synagis and Hendra virus vaccine, vaccines to prevent recurrence of breast and ovarian cancer.
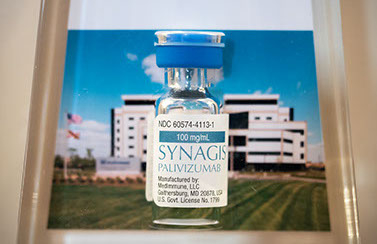
SYNAGIS®
Synagis® is the first successful monoclonal antibody to combat the infectious disease RSV, a major cause of respiratory illness in young children responsible for 57,000 hospitalizations each year. The drug represents the culmination of over 20 years of research at USU and was developed through successful collaboration with, and licensing to Medimmune, Inc. This single license agreement has accounted for approximately $11 billion of total cumulative sales, and accounted for nearly 83% of sales resulting from DoD license agreements.
Synagis featured in Nature Magazine >
SHIGA TOXIN DETECTION
Shiga toxin-producing Escherichia coli (STEC) are responsible for approximately 265,000 intestinal infections annually in the U.S., usually through food-borne outbreaks. Such infections can result in acute diarrhea, hemorrhagic colitis, and in 5–10% of cases, life-threatening hemolytic uremic syndrome. Diagnosis, and thus treatment, was time-consuming, taking 24 hours or more to complete. Researchers at USU addressed this key challenge and developed rapid tests to detect and differentiate the toxins in about 30 minutes, enabling faster diagnosis and implementation of treatment. These diagnostics were licensed to Alere, and are now marketed as Shiga Toxin ChekTMand Shiga Toxin Quik ChekTM, providing actionable results to help optimize patient treatment pathway within 10–30 minutes.
Recipient of 2015 Excellence in Technology Award >
CONTROL OF BLEEDING
Collaboration and licensing arrangements between USU and St. Teresa have led to the product SURGICLOT®, a dissolvable dressing that leaves behind the essential human clotting proteins, fibrinogen and thrombin, at the injury site. SURGICLOT® has been subject to human trials in the UK, Norway, and India and is currently awaiting certification marking (CE Mark) in Europe. SURGICLOT® hopes to become the treatment of choice for cancellous bone bleeding, particularly since the SURGICLOT® shrinks as it works as opposed to other hemostatic agents that expand as they work.
Recipient of 2017 Excellence in Technology Award >
PROGRAM CONTACT INFORMATION
- If you are a commercial business and want to help commercialize USU products, please contact us at orta@usuhs.edu.
- If you have technology transfer, commercialization, or intellectual property questions, please contact us at orta@usuhs.edu.